(1944-2001)

Don Wiley changed how we picture molecular organization at the cell surface. Indeed, he helped to create how we picture it. His two monumental contributions, the structures of influenza virus hemagglutinin in its various states and the structures of class I and class II MHC molecules in combination with peptides, superantigens, and T cell receptors, redefined molecular virology and immunology. He was one of the pioneers who transformed the specialty then known as protein crystallography into the discipline we now call structural biology.

Don burst upon the scene of protein crystallography in 1971, at the landmark Cold Spring Harbor Symposium on Protein Structure. Fresh from the triumph of a 5.5 Å structure of aspartate transcarbamoylase (ATCase) and a recently completed PhD thesis, he arrived from Cambridge in a newly acquired sports car. He continued to cut a dashing figure throughout the meeting. He had been recruited (from Tufts) to the Biophysics graduate program at Harvard by Donald Caspar, who remained an important intellectual influence, but he was ultimately lured by the ATCase project to pursue thesis research in William Lipscomb’s laboratory in the Department of Chemistry. In 1967, the structural basis of allosteric regulation was a major (and contentious) problem. It represented the next level of structural complexity, beyond the three or four simple enzymes for which atomic resolution models were then at hand. By 1971, Don had changed all that, not yet by fully solving the problem of course that took another two decades of work in Lipscomb’s laboratory but by defining the route to an answer.
I first met Don shortly after he arrived at Harvard. I was a student in Caspar’s laboratory in the so-called Jimmy Fund Building of the Children’s Cancer Research Foundation, and Don did an extended rotation there. He carried out diffraction experiments on lipid phases, perhaps nucleating his career-long interest in membranes. We collaborated while he was a graduate student, working out how to use the newly introduced rotating-drum film scanners to index and integrate precession and, later, oscillation photographs. We were recruited by the Department of Biochemistry and Molecular Biology in the same year, and when Don accepted his appointment, a few months after I accepted mine, he came to propose that we set up our laboratories together. Sitting in my tutor’s room in Lowell House, we fantasized how would we build up what we called structural molecular biology (a phrase we had learned from Caspar and Carolyn Cohen) amidst the informational molecular biology then reigning in Cambridge, Massachusetts.
Don joined the Harvard faculty immediately upon completing his degree an unusual step that circumvented the conventional postdoctoral route. The lack of a transitional period, within which to find a worthy research goal, was the source of considerable stress, as Don recounted in an interview with Sondra Schlesinger (see http://virologyhistory.wustl.edu/wiley.htm). For Don was not a person to consider, even for a moment, a bread-and butter project to back up his more ambitious strivings. His determination never to waste time on something ordinary was probably reinforced by the influence of Jim Watson, an intense scientific presence in the Harvard of the 60s and 70s. In any case, by 1974, he had found a direction that would dominate the rest of his career, by seizing upon viral surface glycoproteins the influenza virus hemagglutinin (HA) in particular as a route toward unraveling the molecular mechanisms of cell-cell recognition. Of Don’s success in turning this part of cell biology into crystallography, Max Perutz once wrote that Don had now done the impossible twice. The first time was, of course, his graduate-student success with ATCase.) .
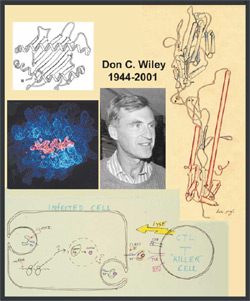
Skehel's discovery led to the second phase of work on HA efforts to define the structural transformations that accompany viral entry. A graduate student, Bill Weis (now at Stanford), obtained the first glimpse of receptor binding in 1987, but it was not until 1994 that two postdoctoral fellows in the laboratory, Fred Hughson (now at Princeton) and Per Bullough (now at University of Sheffield) revealed the remarkable transformation that HA undergoes at low pH. Many of us did not believe, before that moment, that proteins could really do such things. It revised our concept of conformational change.
By the late 1970s, Jack Strominger's laboratory at Harvard had found ways to prepare significant quantities of the HLA major histocompatibility antigen, originally discovered as the principal determinant of transplant rejection,
but already by that time known to be a crucial component of the T cell-mediated immune response. Don’s progress in studying influenza HA made him the obvious collaborator in an effort to visualize HLA. Pamela Bjorkman, a graduate student (now an HHMI Investigator at Caltech), undertook to crystallize it and to determine its structure. The outcome in 1987 was a turning point in immunology. Various lines of evidence had led to the notion that major histocompatibility molecules like HLA would bind antigen-derived peptides and present them on the cell surface. The notion of presentation was still rather vague, however, and a picture was essential, not just to prove the notion but also to work out how to refine it. The structure did just that. For sitting between two ?-helical rails on the outer surface of the molecule was the now famous extra density, promptly ascribed to bound antigenic peptide. Results from many laboratories then came thick and fast, characterizing bound peptides and establishing the principles of their incorporation.
Seeing the extra density was definitely a discovery, and it strongly influenced Don’s scientific directions. During the decade and a half since the 1987 paper, he and his laboratory turned that first glimpse of what the T cell receptor really sees into an entire library of concepts and images. Working with Don, Dean Madden (now at Dartmouth) analyzed the structural principles of peptide binding by class I MHC proteins; Ted Jardetzky (now at Northwestern), Larry Stern (now at MIT), and Jerry Brown (now at Brandeis) determined the structures of class II molecules; and David Garboczi (now at NIH) and Partho Ghosh (now at UCSD) finally crystallized T cell receptor complexes and could look at MHC molecules as the TCR sees them. Recognition and prizes came in abundance (the Louisa Gross Horwitz, Gairdner, Lasker, and Japan Prizes, to name a few), but Don remained strikingly unaffected by the glamor, his thoughts still fixed on what discoveries might come next.
The year 1987 marked not only the revolution brought about by the HLA structure, but also a transition in the research group. The shared laboratory that Don and I had founded was adopted by the Howard Hughes Medical Institute, and together we established a second laboratory in the HHMI Unit at Children’s Hospital almost a return to our common roots in the old Jimmy Fund Building, one block away. The HHMI transition allowed both of us to expand our scientific scope and to undertake even riskier projects. One example was our joint resolve to contribute what we could to the general effort to understand HIV. I believe that a particularly fulfilling moment for Don came when Winfried Weissenhorn (now at EMBL) worked out the structure of a truncated form of the HIV-1 gp41 trimer in the conformation it adopts after the fusion-activating transition. I recall how Don told me on the phone that the structure was at last done and that there might be a discovery the N and C termini of the gp41 ectodomain were adjacent in space after the transition. He believed that this would be true for influenza HA (Jue Chen later showed that it was), but the Hughson-Bullough structure had not quite established it. The implication was that these fusion proteins were instruments for forcing two bilayers into proximity and that the function of the conformational change was to squeeze them together.
I have said that Don’s view of significant scientific observations was embodied in his use of the word discovery; his integration of discoveries into a picture of external reality was communicated through the simplicity of a cartoon. Clear, well-established notions, such as the way a T-killer cell recognizes the antigen presented by its target, could be summarized in a few drawn lines and a few short words. Don hated obfuscation or irrelevant detail; the little Etc in the drawing here probably wraps up more than half the papers in J Exp. Med. during the last decade! The mesmerizing charm of this cartoon also encapsulates some of Don’s intellectual and personal charisma. Four o’clock teatime in the laboratory became an institution when we were still both crammed into about a thousand shabby but wonderful square feet in the now vanished Gibbs Laboratory at Harvard, and continued when we acquired our own little tea room in the new Fairchild Building. When in the right mood, Don could hold forth at teatime for an hour or more, with his laboratory members (and mine) arrayed around him like iron filings near a magnet. At such moments, and at scientific conferences, he had a larger-than-life presence, enhanced by his chiaroscuro affectation of wearing nothing but black or black-and-white. He was a superb mentor of graduate students, and from 1980 to 1992 he chaired the Biophysics program that had once granted him a PhD.
To two former postdoctoral fellows, now married to each other, Don wrote recently on the occasion of the birth of their first child: This is the beginning of a great adventure. I am not sure that anything changes your life more than a child. Similarly, there is nothing more fun or interesting. When Don married Katrin Valgeirsdottir, he learned Icelandic, and Iceland became an adopted second home. He believed in family, and cared very much about his own. He was also deeply conscious of his scientific family his current and former students and postdoctoral fellows, his collaborators, his mentors. I would wander into his office at the end of almost every day that we were both in town, to talk about our science and to seek or give advice. These conversations were often terminated only by a phone call from home, presumably announcing dinner. I miss intensely those remarkable encounters, just as the rest of Don’s extended scientific family mourns the loss probably larger than any of us yet realizes of his powerful yet unpretentious intellectual presence.
– Stephen C. Harrison

- Don Wiley Resources:
- » Wiley Oral History
- » MCB Tribute
- » Harvard Statement
- » Guestbook
