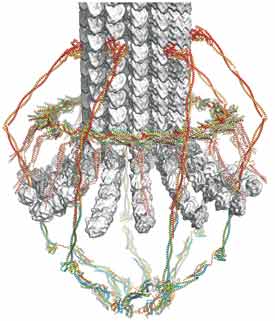
Molecular organization of kinetochores
Kinetochores link chromosomal centromeres to microtubules of the mitotic spindle. They are both critical architectural elements in cell division and centers for integrating relevant cell-cycle signals. Kinetochores in budding yeast are assemblies of over 50 distinct protein species, nearly all of which have homologs in other eukaryotes. They connect a single centromeric nucleosome with a single spindle microtubule and thus appear to represent a single module of the more complex, parallel-module organization in most other species. The kinetochore components come together into well-defined protein complexes, which have an elaborate network of regulated contacts with each other, with centromeric chromatin, and with microtubules. It is useful to describe them as microtubule-proximal complexes, chromosome-proximal complexes, and intermediate adaptors. We have undertaken to build up a three-dimensional picture of a yeast kinetochore, starting with x-ray crystallographic studies of its key constituent protein complexes and now with cryo-EM structures of much larger subassemblies (see Hinshaw and Harrison, 2017; Jenni et al, 2017).
The key microtubule-proximal components in budding yeast are a 600 Å-long, rod-like element, known as the Ndc80 complex (Ndc80c)(Wei et al, 2005) and a heterodecameric assembly, the DASH/Dam1 complex, which further oligomerizes into a ring around the microtubule (Miranda et al, 2005). A structure of the ring, determined by cryo-EM, together with data on interactions between the Ndc80c and the DASH/Dam1c and between each of these elements and a microtubule, gives us a picture of the microtubule-attachment apparatus (Jenni and Harrison, 2018) and allows us to ask experimental questions about the mechanism by which kinetochores sense tension from bipolar spindle attachment.
The principal chromatin-proximal components in budding yeast are — in addition to the centromeric nucleosome, which has a distinct histone H3 known as Cse4CENP-A — a dimer of Mif2CENP-C and the 13-component Ctf19 complex (Ctf19c), corresponding to the so-called “constitutive chromatin associated complex” (CCAN) in most multicellular eukaryotes. Our recent cryo-EM structure of the Ctf19c (Hinshaw and Harrison, submitted) allows us to model most of the chromatin-proximal assembly and to integrate into it earlier studies of some of its component structures (Schmitzberger and Harrison, 2012; Hinshaw and Harrison, 2013) and of the mechanism by which it directs and regulates cohesin loading at the centromere (Hinshaw et al, 2015; Hinshaw et al, 2017).
The key adaptor that connects chromatin-proximal with microtubule-proximal components is the rod-like MIND complex (Mis12 complex in metazoans)(Dimitrova et al, 2016; Petrovic et al, 2016). It interacts at one end with both Mif2CENP-C and with the Ame1 subunit of Ctf19c and at the other with one end of the Ndc80c. Like most of the connections between the various kinetochore subcomplexes, specific phosphorylation events control the timing and stability of these interactions.